What Are Enzymes | History, Structure, Function 2024
Table of Contents
Enzymes are proteins that speed up reactions by acting as catalysts. They increase the rate of reaction without being consumed or permanently altered by themselves.
In Greek enzymes are divided into sections “En” which means “in” and the second part is “zyme” which means living. The study of enzymes is known as enzymology. (Enzyme Definition)
What Are Enzymes | Delving Deeper In The History Of Enzymes
The physical nature of life is based on the making and breaking of differently composed at all levels involved in the metabolism.
Thousands of compounds must be shaped to produce the organelles and other structures present in living organisms.
The formation may involve the making of large molecules from smaller molecules or vice versa. Moreover, all these reactions should take place at moderate temperatures and with a speed good enough to support life.
It is a common laboratory observation that reactions take place within a cell. If accomplished outside the cell needs a very high temperature and there is no control over the speed of the reaction.
Why do the same reactions need different environments to accomplish when taking place inside the cell? What is the missing connection between the living and non-living environment?
Initially, people thought that it is because of life itself. A living thing can do what cannot be done by non-living conditions.
History Of Enzymes
The enzymes were first discovered by a French chemist named Anselme Payen. He called them diastase in 1833.
A few decades later, Louis Pasteur while studying the fermentation of sugar to alcohol by yeast discovered that there is an essential force, which he called ferments that only functions within living organisms.
The term enzyme was first used in 1878 by Wilhelm Kuhne, a German physiologist. Thus, after a lot of hard work, the scientists were able to find the missing connection.
They were able to demonstrate that it is not life but a large biomolecule made of amino acids, which behaves as a catalyst in the functioning of the cell.
These large molecules not only speed up the reactions but also make them happen at a moderate temperature which can support life. This biomolecule takes part in all the formations and breakdown of compounds that follow specific pathways.
All the reactions require the biomolecule as a definite controlling system. The control should be for the reaction’s direction, speed, and specificity.
Cells control all operations by producing the proper biomolecule, named Enzymes. Enzymes are proteins that are always produced in the proper amounts, at the appropriate time, and at the proper place where they are needed.
And according to research, different factors affect enzyme activity i.e., pH, Temperature, Enzyme and Substrate Concentration, etc.
As mentioned above in the enzymes definition, they are required by the biomolecules, because nearly all chemical reactions of life are so slow without catalysts that they cannot sustain life.
The enzymes generally speed up reaction rates by factors between 108 and 1020 and thus made the life process possible.
Classification Of Enzymes
Enzymes Are Specific
More than 5,000 different enzymes have been discovered in living organisms, and the number grows as research continues. The most important properties of enzymes, so far studied are their specificity.
Each enzyme acts on a single substrate (reactant) or a small group of closely related substrates that have virtually identical functional groups that are capable of reacting.
Nomenclature Of Enzymes
In the beginning, when the data regarding the enzymes were very limited each enzyme was given a trivial name. For example, pepsin, trypsin gastrin, etc.
As the data increased a need for some rule was badly felt. Thus, all enzymes were named according to a standardized system.
The Suffix Ase
All enzymes were named to end in the suffix ‘ase’. These names characterize the substrate or substrates acted upon and the type of reaction catalyzed.
For example, cytochrome oxidase, an important respiratory enzyme, oxidizes (removes an electron from) a cytochrome molecule. Malic acid dehydrogenase removes two hydrogen atoms from (dehydrogenates) malic acid.
These common names, although conveniently short, do not give sufficient information about the reaction catalyzed. For example, neither identifies the acceptor of the removed electron or hydrogen atoms.
The International Union of Biochemistry 1961 lists longer but more descriptive standardized names for all well-characterized enzymes.
As an example, cytochrome oxidase is named cytochrome c: O2 oxidoreductase, indicating that the particular cytochrome from which electrons are removed is the c type and that oxygen molecules are the electron acceptors.
Malic acid dehydrogenase is called L malate: NAD oxidoreductase, indicating that the enzyme is specific for the ionized L form of malic acid (malate) and that a molecule abbreviated as NAD is the hydrogen-atom acceptor.
Classes Of Enzymes
A systematic approach with new names was first suggested in 1965 and revised n 1972. According to this system, all enzymes are grouped into six main classes.
The main classes of enzymes and the type of chemistry they participate in are given below.
Classification Of Enzymes: 6 Classes Of Enzymes
| Main Class | Types Of Reaction Catalyzed |
|---|---|
| Oxidoreductase | Oxidation-reduction of all types. |
| Transferase | Transfer of an intact group of atoms from a donor to any acceptor molecule. |
| Hydrolases | The hydrolytic (H2O participates) cleavage of bond. |
| Lyases | The cleavage of bonds by means other than hydrolysis or oxidation. |
| Isomerases | Inter-conversion of various isomers. |
| Ligases | The bond formation is due to the condensation of two different substances, with energy provided by ATP. |
The system is designed to zero on the specific identity of each enzyme by dividing each main class into subclasses and sub-subclasses.
By using a numbering system throughout the scheme, each enzyme can be assigned a numerical code. Such as 2.1.3.4, where the first number specific subclass and sub-subclass respectively and the final number represents the serial listing of the enzyme in its subclasses.
For example, histidine decarboxylase (traditional name) is identified as histidine decarboxy-Iyase, 4.1.1.22; alcohol dehydrogenase as alcohol: NAD oxidireductase, 1.1.1.1; urease as urea amidohydrolase, 3.5.1.5.
Let us see how it works,
| No | Lyases | Cleavage | Class |
|---|---|---|---|
| 4.1 | Carbon-carbon Iyases | Cleavage of C-C bonds | Subclass |
| 4.1.1 | Carboxyl-Iyases | Cleavage of C-COO- bond | Subclass |
| 4.1.1.22 | Histidine carboxylase | Cleavage of C-COO bond in histidine | Name |
| 4.1..2 | Aldehyde-Iyases | - | - |
| 4.2 | Carbon-oxygen lyases | Cleavage of C-O bond | - |
| 4.3 | Carbon nitrogen Iyases | Cleavage of C-N bond | - |
| 4.4 | Carbon-Sulphur Iyases | Cleavage of C-S bond | - |
| 4.5 | Carbon halogen Iyases | - | - |
| 4.6 | Phosphorus-oxygen Iyases | - | - |
The use of this modern classification of enzymes is required in professional research journals. However, the older system is still widely practiced, mostly in monographs and textbooks.
Structure And Characteristics of Enzymes
One of the basic qualities of enzymes is that they are specific in action.
If we elaborate on the specificity, we will understand that specificity is because of their power of recognizing the substrate from a sea of molecules.
How do they do this? They do this because of the specific structure of the substrate.

This means that enzymes are structure-specific in nature. This structure specificity of the enzymes is based on their own specific structure.
When proper substrate colloids with a proper enzyme that has proper structure, they fit in to carry out the phenomena of catalysis.
Thus, for a proper understanding of Enzymes and enzyme action, we need to understand their structure and chemical nature.
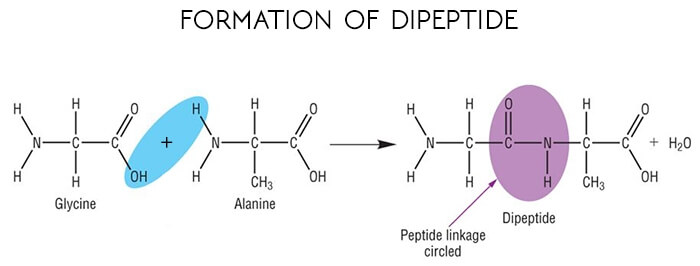
Enzymes are proteins, which are giant and of a special type, that are composed of hundreds of amino acids. These amino acids are joined together linearly to produce chains.
The union of amino acids into polypeptide chains of proteins occurs through peptide bonds involving the carboxyl group of one amino acid and the amino group of the next amino acid.
Homopolymers And Heteropolymers
These chains coil upon themselves to form a globular three-dimensional structure. These structures may be the result of a single chain of amino acids or more than one chain may give rise to these structures.
The polypeptide chains may be identical and thus known as homopolymers (homo-same; poly-many; mers-parts). Mostly the chains in enzymes are different. In such cases, they are known as heteropolymers (hetero-different; poly-many; mers- parts).
Apoenzyme And Cofactor
About 90 % of the Cofactor enzymes are pure protein i.e. composed of amino acid chains only.
In the rest of the 10%, the enzyme protein is the central part associated with a small non-proteinous part.
In such enzymes, the protein parts are known as Apoenzyme, and a much smaller, organic, non-protein portion is called the Cofactor.
What Is Holoenzyme?
Apoenzyme and co-factor join together to form a functional enzyme which is known as Holoenzyme.
The cofactor is essential for the catalytic activity of the holoenzyme. The Cofactor can be further divided based on their attachment with apoenzyme into three categories.
What Is The Difference Between A Coenzyme And A Prosthetic Group?
A cofactor is called a prosthetic group when it is tightly attached to the apoenzyme by covalent bonds.
Sometimes the cofactor is loosely attached to the apoenzyme through weak forces. In this status, the non-proteinaceous part is called coenzymes.
Metal Activators
The cofactor may still be a metal ion such as magnesium, and potassium for their activity. The metal ions are often called metal activators. For example, Hexokinase requires Mg++ ions.
Active Site of Enzyme
Most of the enzymes are composed of about 250 amino acids. As the attachment of amino acids grows the peptide chain length increases. Dipeptide changes to tripeptide, tripeptide into tetrapeptide, and so on.
Every added amino acid increases the chain length but this does not mean that it gives rise to a straight structure. Instead, the chain folds and bends upon itself to give rise to a globular three-dimensional structure.
The resulting three-dimensional structure of an enzyme which is a functional unit always has depression or groove on its surface which is known as the active site of an enzyme.
This active site is mostly composed of about 10 to 12 amino acids brought close to each other by bending and folding the polypeptide chain.
The rest of the amino acids play a part in stabilizing the enzyme structure even during enzyme action. The active site engages the substrate for enzyme action during enzyme catalysis.
Types of Active Site
The active site is further divided into types:
- Binding site
- Catalytic site
The binding site recognizes the substrate and helps in the binding of the substrate/inhibitor. While the catalytic site when engages does the catalysis. And from inhibitors, comes the process of enzyme inhibition.
Enzyme Action
The enzyme action mechanism involves the making or breaking of biomolecules. The enzyme’s role in the reaction is of the biocatalyst.
All the reactions, which can otherwise take place if provided with an enzyme, simply increase their speed. The enzymes can speed up the reaction rate but they cannot force a reaction to take place.
The mechanism involved in the reaction remains the same enzymes only speed up the process. How it is possible?
To understand this, we have to first understand how the reaction takes place and then how enzymes speed up the process.
The process of making and breaking (reaction) depends upon the change in energy level. In a chemical reaction usually, the most energetic molecules can undergo chemical reactions.
Such molecules become more energetic than others of the same kind by being subjected to different numbers and types of collisions. A rise, in temperature, increases the possible collision rate thus reaction rate is increased.
Energy Of Activation
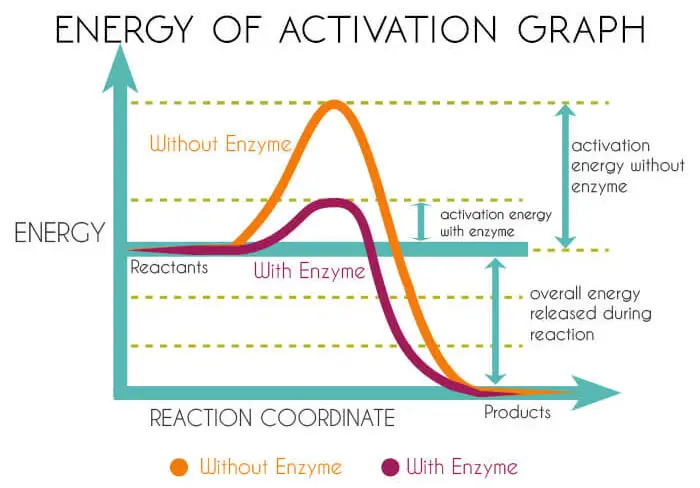
Molecules that have enough energy to undergo a reaction and are converted to products. The energy required for the molecules to change is known as the energy of activation.
In laboratory experiments, energy is provided to the system to increase the activation energy of the molecules to produce products.
But in the living system, it is not possible to increase the temperature to such an elevated state of energy, which could not support life.
The only possibility to produce products without increasing the energy of the molecule is to decrease the activation energy of the system.
This is possible only because of the mediation of enzymes. It is through enzymes that life carries out such reactions at a relatively low temperature suitable for life.
The enzymes being proteins do this job by lowering the energy of activation. So that a much greater fraction of the substrate molecules have sufficient energy to react without a temperature increase.
Thus substrates are converted into products. A careful study of graphs can further help in the understanding of this enzyme action mechanism.
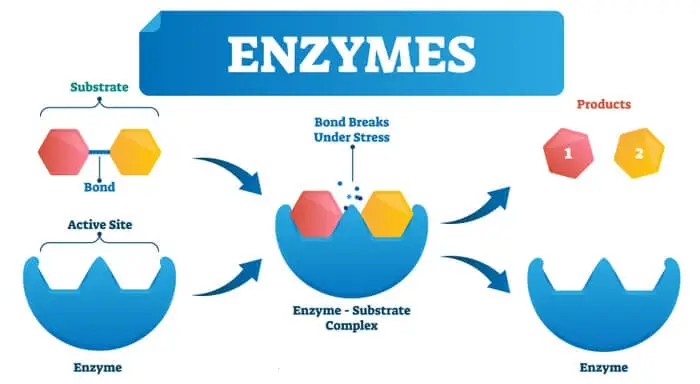
Enzyme Substrate Complex
For an enzyme to decrease the energy of activation during the reaction, the enzyme must combine temporarily with the substrate or substrates to form an Enzyme Substrate Complex.
E + S → ES Complex → E + P
E = Enzyme
S = Substrate
ES Complex = Enzyme Substrate Complex
P = Products
The formation of the enzyme-substrate complex plays a key role in the reduction of the activation energy of the reaction.
The enzyme-substrate complex thus formed decreases the activation energy by changing the status of the substrate.
At this point, we should not forget that the substrate binds with the enzyme only at the active site, which is not a flat surface but a grove.
Once substrate combines to form an enzyme-substrate complex. The resulting structure is neither enzyme nor substrate but complex.
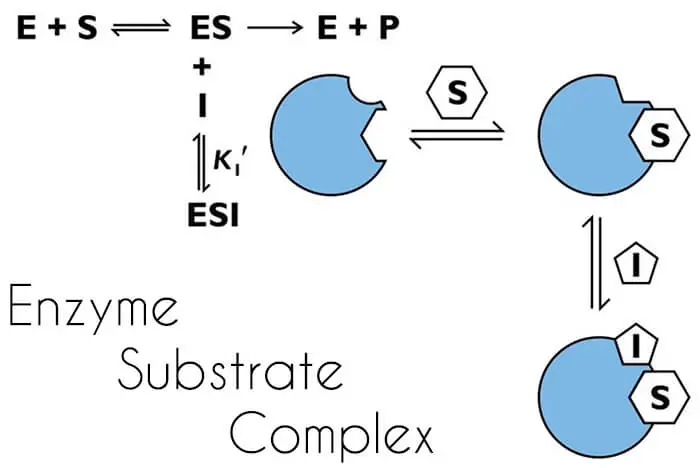
In the groove, the micro-environment is modified to the extent that forces stabilizing the molecules (substrate and enzyme) within this complex are modified.
Thus, it affects the forces of the substrate, causing some of the substrate bonds to break; then the bonds rearrange to form a stable structure of the products, much more rapidly than in the absence of the enzyme.
Enzyme molecule remains stable only because of their size, which is about a hundred times larger than the substrate.
Bonding Involved In The Formation Of ES Complex
The kinds of bonds between enzymes and substrates can be covalent, ionic, hydrogen, and Vander Walls. The covalent and ionic bonds are most important concerning the energy of activation for a reaction.
But the more numerous hydrogen bonds and Vander Walls interactions contribute to the structural orientation of the enzyme-substrate complex.
Even when strong covalent bonds are formed, they are usually very rapidly broken to release new product molecules.
Both covalent and non-covalent bonds between enzyme and substrate are formed between parts of the R portions of amino acid residues in the enzyme, not between the carbon and nitrogen atoms involved in the peptide bonds.
Thus, enzymes must have the proper amino acids (composition) and must have them in the right places (sequence) for their proper functioning.
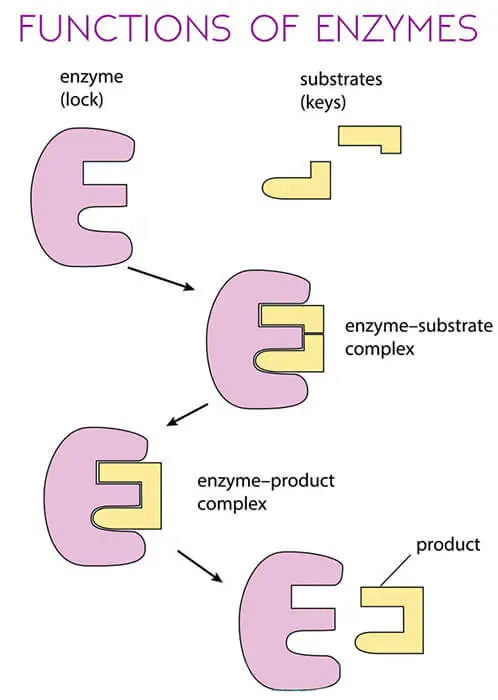
Function of Enzymes
The points and diagram below will simplify the function of the enzyme.
- They speed up reactions and act as catalysts. As they make reactions millions of times faster.
- Help in digestion, Breaking large complex molecules into smaller molecules i.e., glucose which is utilized by the organism’s body.
- Helps in unwinding the DNA and copying it inside the cell.