Define Enzyme Inhibition & Types Of Enzyme Inhibition 2024
Table of Contents
Enzyme inhibition or inhibitors are one of the crucial factors that affect enzyme activity.
Enzyme Inhibitors Definition: Those chemical substances which can react in the place of the substrate with an enzyme but are not converted into products and block the active site temporarily or permanently are called inhibitors.
Enzyme Inhibition Definition: Enzyme inhibition is the capacity of the inhibitors to retard or stop enzyme catalysis. Substrates for example poisons, anti-metabolites, antibiotics, and some drugs act as inhibitors for certain enzymes and thus stop their activity.
Types Of Enzyme Inhibition And Inhibitors
There are two types of inhibitors.
- Irreversible inhibitors
- Reversible inhibitors
Reversible Vs Irreversible Inhibition
Below is a table of reversible vs irreversible inhibition.
| Factors | Irreversible Inhibition | Reversible Inhibition |
|---|---|---|
| Binding Through | Covalent Bonding | Non-Covalent Bonding |
| Enzyme-Inhibitor Complex | Dissociates Slowly | Dissociates Quickly |
| Enzymatic Action | Restores | Takes Longer Time |
| Types | Competitive, Noncompetitive, Uncompetitive, And Negative Feedback | Takes Place Through Covalent Inactivation Of Enzymes Active Site |
| Examples | Anti-viral Drugs (Tipranavir, Ritonavir), DHFR | Disinfectants, Insecticides, Herbicides, DFMO, DFP |
What is Irreversible Inhibition?
In this type of inhibition, the inhibitor checks the reaction rate by occupying the active site or destroying the enzyme’s globular structure.
They occupy the active site directly by having a covalent bonding to any of the amino acids situated in the active site or they may physically block the active site.
The irreversible inhibitor may also combine with sulphydryl (-SH) groups and destroy the disulfide bridges, which are essential for maintaining the spherical structure of the enzyme. As a result, the enzyme molecule is denatured.
What is Reversible Inhibition?
In reversible inhibition, the inhibitor reacts with the active site temporarily. They form weak interactions and either retard or stop the enzyme activation.
The effect of reversible inhibitors can be neutralized completely or partly by an increase in the concentration of the substrate.
Types Of Reversible Enzyme Inhibition
Four major types of reversible inhibitors (inhibition) are known to exist. They are:
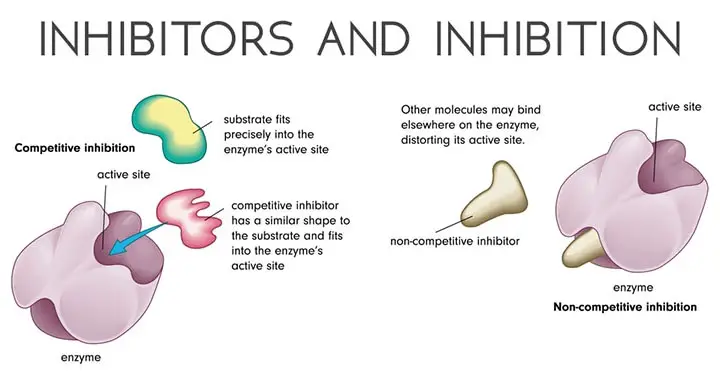
- Competitive Inhibition
- Noncompetitive Inhibition
- Uncompetitive Inhibition
- End Product Inhibition (Negative Feedback)
Competitive Vs Noncompetitive Vs Uncompetitive Inhibitors
Below is a summarized table for competitive vs noncompetitive vs uncompetitive inhibitors.
| Factors | Competitive Inhibitor | Noncompetitive Inhibitor | Uncompetitive Inhibitor |
|---|---|---|---|
| Binding | Only To Free Enzyme | To Both Free Enzyme & Enzyme-Substrate Complex | Only To Enzyme-Substrate Complex |
| Binding Duration | Dissociates From Enzyme Within Short Period | Remains Binded For Considerable Time Period | Takes Longer To Leave |
| Occurrence | In The Active Side | Outside The Active Side | Outside The Active Side |
| Active Site | Blocks The Active Site | Alters The Shape/Size Of Active Site | Doesn't Alters The Active Site |
| Substrate Concentration | Increases The Concentration | Does Not Change | Reduces The Concentration |
Competitive Vs Noncompetitive Inhibitors
Competitive and noncompetitive enzyme inhibitors differ with respect to:
| Factors | Competitive Inhibitor | Noncompetitive Inhibitor |
|---|---|---|
| Shape/Structure | Similar To Substrate | Different To Substrate |
| Binding Site | To The Active Side | Bind To E-S Complex |
| Binding Duration | Dissociates From Enzyme Within Short Period | Remains Binded For Considerable Time Period |
| Active Site | Blocks The Active Site | Alters The Shape/Size Of Active Site |
| Substrate Concentration | Increases The Concentration | Does Not Change |
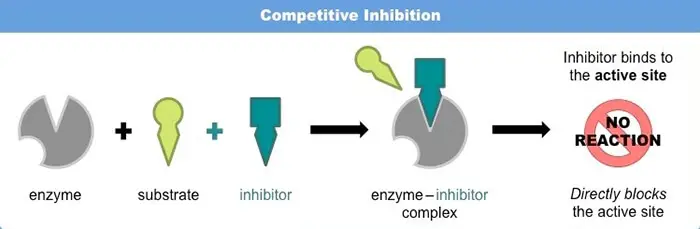
A) Competitive Inhibition
Because of their structural similarity with substrate they are associated with enzymes at the active site. They can attach themselves at the binding site but cannot activate its catalytic site, thus giving off.
The degree of inhibition depends upon the relative concentration of substrate and inhibitor. By increasing substrate concentration reaction rate can be increased, thus the effect of an inhibitor can be reduced to almost zero.
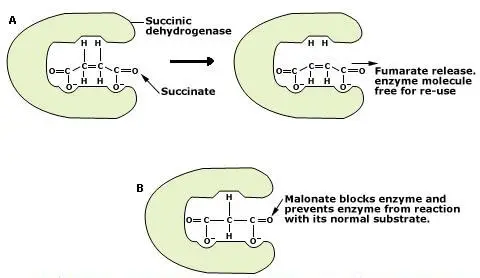
Malonic acid and Succinic acid are very good examples, which compete for the same site (Fig.5.11).
B) Noncompetitive Inhibition
In this type of inhibition, the inhibitors do not compete with the substrate for the attachment on the active site. Their function is to form the enzyme inhibitor complex at a point other than the active site.
They alter the structure of the enzyme in such a way that even if a genuine substrate tries to bind the active site, catalysis fails to take place.
As substrate and inhibitor do not compete for the same site, an increase in substrate concentration does not affect the reaction rate.

C) Uncompetitive Inhibition
These Inhibitors always combine with the Enzyme Substrate Complex and not with the enzyme when the enzyme is alone. As a result, the reaction rate is inhibited. This inhibition is also not affected by an increase in substrate concentration.
D) End Product Inhibition (Negative Feedback Inhibition)
This inhibition is caused by the interaction of a product with an enzyme in the sequence of its formation.
In feedback inhibition, the inhibited enzymes may very often be an allosteric enzyme (allo means “other”; that is, different from the active site). This type of inhibition is mostly of competitive type (see the below diagram).
How Does End Product Inhibition Work?
We have mentioned that numerous foreign ions or molecules (inhibitors) can inhibit enzymatic action, in most cases by altering the configuration of the enzyme so that it cannot effectively form a complex with the substrate.
However normal cellular constituents can also alter several enzymes thus resulting in decreases or increases in their functions.
Such effects are important mechanisms for homeostatic control at the metabolic level. They help organisms produce only the proper amount of the compounds they need.
The most common case is the inhibition of a particular reaction by a metabolic that is chemically unrelated to the substrate with which the enzyme reacts.
To understand this, consider an example in which compound A is converted by a series of enzymatic reactions via intermediates B, C, D, and E to an essential product F.
After this number of reactions, compound F no longer bears much structural resemblance to A.

Nevertheless, F can sometimes reversibly combine with the first enzyme to inhibit its combination with A. This is an example of feedback inhibition or end-product inhibition.
Its advantage is that it provides a rapid and sensitive mechanism to prevent over synthesis of compound F. Because the feedback inhibition occurs only after F has built up to a level that is sufficient for cellular needs.
Later, when the amount of F in the cell has been reduced (by incorporation into a structural component of the cell), F molecules dissociate from enzyme number 1 and allow it to become active again.
Cases of feedback inhibition nearly always involve the action of a product of a metabolic pathway upon the first enzyme of that pathway.
Example Of End Product Inhibition
A well-studied example of feedback inhibition in plants occurs in the formation of the nucleotide uridine monophosphate (UMP), which begins with aspartic acid and carbamyl phosphate.
The pathway requires five enzyme-catalyzed steps, but only the first enzyme, aspartic transcarbamylase, is susceptible to feedback control by UMP; no other reaction is blocked similarly by reactants and products in the pathway.
What Are Allosteric Enzymes And Allosteric Sites?
Enzymes that combine with and respond (either negatively or positively) to small molecules such as F are called allosteric enzymes. The sites at which the combination takes place with the smaller molecules are called allosteric sites.
Enzyme Inhibition Questions
Q: What Enables Competitive Inhibitors To Bind To A Specific Enzyme?
Ans: The thing that enables competitive inhibitors to bind to a specific enzyme is their resembling structure to that enzyme’s substrate.
Q: How Do Competitive And Noncompetitive Enzyme Inhibitors Differ?
Ans: The competitive inhibitors actually bind to the active site and prevent the substrate from binding. They block the active site. While the noncompetitive inhibitors bind to other sites (E-S Complex) than the active site. They alter the shape/size of
Q: What Are The Two Important Binding Sites Of Allosteric Enzymes?
Ans: The two important binding sites for allosteric enzymes are the substrate binding site and the effector binding site.