Mineral Nutrition Types, Deficiency, Symptoms 2024
Table of Contents
Mineral nutrition and knowledge about its all aspects is an important subdivision of plant physiology. It deals with the phenomena which make us understand how elements are absorbed by a plant and what role they play in the survival of the plant.
Experimentations done so far with mineral nutrition have greatly improved humans’ understanding of agriculture, forestry, and other utilization of plants for human benefits.
The process of understanding is going on for the past one hundred and fifty years. Still, more improvements are required to realize the important role played by mineral nutrition.
What Are Essential Elements?
Those elements which are required by plants for proper growth, development, and maintenance are called essential elements.
Criteria for Essentiality of Mineral Elements
To judge essential or non-essential elements of any plant there are two principal criteria used.
First Criteria: An element is essential if the plant cannot complete its life cycle in the absence of that element.
Second Criteria: An element is essential if it forms part of any molecule or constituent of the plant that is itself essential in the plant (for example, nitrogen in proteins and magnesium in chlorophyll).
Third Criteria: The 3rd criteria were developed by Daniel Arnon and Perry Stout (1939). This is not widely accepted but some workers accept it.
They suggested that an essential element must be acting directly inside the plant, and not helping some other element to be more readily available in the plant or disturb its effect.
Methods for Detection of Mineral Nutrients
Below are the methods for detecting mineral nutrients in plants:
Dry Plant Matter Method
In this method, freshly harvested plants or plant parts are heated to 70 to 80°C for a day or two. In the procedure, nearly all the water is driven off. The remaining material called Dry Matter is analyzed for its components.
Principal components of dry matter are:
- Cell-wall, like polysaccharides and lignin,
- Protoplasmic components, like proteins, lipids, amino acids,
- Organic acids, malic acid, citric acid, etc.,
- Certain elements such as potassium exist as ions but form no essential part of any organic compound.
At least 60 elements have been discovered in plants, including gold, lead, mercury, arsenic, and uranium.
Atomic Absorption Spectrometer Method
This technique is used to measure the concentrations of elements in plants. In the past 20 years, this method has been improved greatly.
To measure metals and some nonmetals, the method now used is the Atomic Absorption Spectrometer. Even more valuable are Optical Emission Spectrometers.
In this method, elements are vaporized at temperatures above 5,000°K. Such high temperatures temporarily excite electrons from their ground-state orbits into higher orbits.
When these electrons move back to their original ground states electromagnetic energy is emitted at wavelengths that are specific for each element.
These wavelengths are measured and the energy is quantified by the spectrometer. Thus, the elements involved can be calculated.
Method of Studying Plant Mineral Nutrition
Solution Culture Method
Starting in about 1804, scientists began to appreciate that plants require calcium, potassium, sulfur, phosphorus, and iron.
In 1860, three German plant physiologists (W. Pfeffer, Julius Sachs, and W. Knop) recognized how difficult it is to determine the kinds and amounts of elements essential to plants growing in a medium as complex as soil.
Nutrient Solution
They, therefore, grew plants with their roots in a solution of mineral salts called Nutrient Solution, the chemical composition of which was controlled and limited only by the purity of chemicals then available.
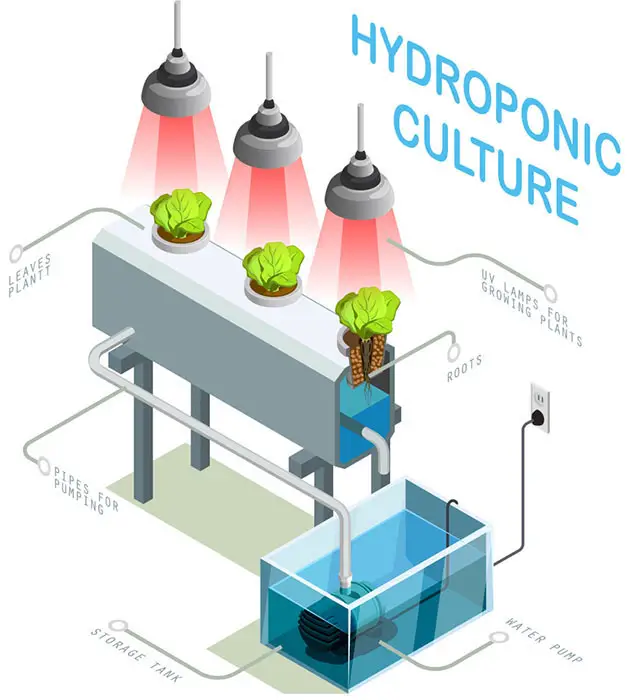
Growing plants in this way are referred to as hydroponics, soil-less culture, or solution culture method. Fig., 6.1.
Although the nutrients requirements of only 100 or so (mostly cultivated) species have been calculated, the nutrient solutions mostly include 13 elements believed to be essential for all angiosperms and gymnosperms. (Table 6.1)
Mineral Nutrition Table
Essential plant nutrients with their relative amounts in plants, functions, and classification:
| Name | Chemical Symbol | Relative % in Plants | Function in Plants | Nutrient Category |
|---|---|---|---|---|
| Nitrogen | N | 100 | Proteins, Amino Acids | Primary Macronutrients |
| Phosphorus | P | 6 | Nucleic Acids, ATP | |
| Potassium | K | 25 | Catalyst, Ion Transport | |
| Calcium | Ca | 12.5 | Cell Wall Component | Secondary Macronutrients |
| Magnesium | Mg | 8 | Part of Chlorophyll | |
| Sulfur | S | 3 | Amino Acids | |
| Iron | Fe | 0.2 | Chlorophyll Synthesis | Micronutrients |
| Copper | Cu | 0.01 | Components of Enzymes | |
| Manganese | Mn | 0.1 | Activates Enzymes | |
| Zinc | Zn | 0.03 | Activates Enzymes | |
| Boron | B | 0.2 | Cell Wall Component | |
| Molybdenum | Mo | 0.0001 | Involved in N Fixation | |
| Chlorine | Cl | 0.3 | Photosynthesis Reactions |
Mineral Nutrition Table 6.1
Status Of Mineral Salt In Plants
The status of mineral salt in any plant can be divided into three categories:
- Deficient Zone
- Adequate Zone
- Toxic Zone
Mineral elements in a relative amount as compared to nitrogen in dry shoot tissue. May vary depending on plant species.
Deficient Zone
This is in the zone which is observed in the range of low nutrient salts (element) concentrations. In it, plant growth is restricted.
Different types of morphological and physiological disorders appear. If more of the element is provided and its concentration in the plant enhances, a dramatic increase in growth is observed.
Adequate Zone
This is the zone above the critical concentration when a further increase in the concentration does not appreciably affect growth.
The adequate zone represents luxury consumption of the element, during which storage in vacuoles occurs. This zone is fairly wide for macronutrients but is much narrower for micronutrients.
Toxic Zone
Continued increases of any element lead to status when the accumulated salt becomes toxic and a decline in growth is observed, which can be averted by the decrease of the element.
Increasing environmental pollution is bringing much more attention to the toxic effects of both essential and nonessential elements thus causing nutrient deficiency in the plants.
Increased accumulation of salts is also a problem in many soils, and many approaches, including genetic tolerance, are being sought to relieve the toxicity.
Types of Nutrients And Their Functions
Micronutrients And Macronutrients
The nutrients needed in tissue concentrations equal to or less than 100 mg/kg of dry matter are often called trace elements or micronutrients. While the nutrients needed in concentrations of 1,000 mg/kg of dry matter are known as macronutrients.
Besides these essential elements, some species also require some other elements. For example, sodium is required by numerous desert species, such as Atriplex vesicaria, common to dry inland regions of Australia.
The Function Of Essential Elements
Essential elements have been classified based on their function into four groups:
- Role in the structure of an important compound,
- Having an enzyme-activating role,
- Playing a role in osmotic phenomena.
- Transient structural roles
Mostly there is no sharp distinction between their functions because several elements form structural parts of essential enzymes and help catalyze the chemical reaction in which the enzyme participates.
All elements in soluble form, whether free or bound structurally to essential compounds, contribute to osmotic potentials.
Thus, aid in the buildup of the turgor pressure necessary to maintain form, speed growth, and allow certain pressure-dependent movements. For example, stomatal opening, and movements of leaves.
Nutrient Deficiency
Nutrient deficiency in plants is a major reason for various types of diseases in plants.
The plants respond to an inadequate supply of an essential element by showing morphological abnormalities popularly known as deficiency symptoms.
Such visually observable symptoms include
- Stunted growth of roots, stems, or leaves.
- Chlorosis (yellow spotting) or necrosis (blackening and death) of various organs.
The deficiency symptoms for any element depend primarily on two factors:
- The function or functions of that element.
- Whether or not the element is readily translocated from old leaves to younger leaves.
Nutrients In Plants Along With Table
Most of the symptoms appear on the plant’s shoot system and are easily investigated. Root symptoms, however, cannot be seen without removing the roots from the soil. That is why root deficiency symptoms have been less recorded.
Characteristic symptoms often help determine the necessary functions of the element in the plant, and knowledge of symptoms helps agriculturists and foresters determine how and when to fertilize crops.
Here below is the table of nutrient deficiency symptoms in plants:
Plants Nutrient Deficiency Table
| Plant Nutrients | Condition | Visual Symptoms |
|---|---|---|
| Nitrogen | Deficiency | Light green to yellow appearance of leaves, especially older leaves; stunted growth; poor fruit development. |
| Excess | Dark green foliage may be susceptible to lodging drought, disease, and insect invasion. Fruit and seed crops may fail to yield. | |
| Phosphorus | Deficiency | Leaves may develop purple coloration; stunted plant growth and delay in plant development. |
| Excess | Excess phosphorus may cause micronutrient deficiencies, especially iron or zinc. | |
| Potassium | Deficiency | Older leaves turn yellow initially around margins and die; irregular fruit development. |
| Excess | Excess potassium may cause deficiencies in magnesium and possibly calcium. | |
| Calcium | Deficiency | Reduced growth or death of growing tips; blossom-end rot of tomato; poor fruit development and appearance. |
| Excess | Excess calcium may cause a deficiency in either magnesium or potassium. | |
| Magnesium | Deficiency | Initial yellowing of older leaves between leaf veins deficiency spreading to younger leaves; poor fruit development and production. |
| Excess | High concentration is tolerated in plants; however, an imbalance with calcium and potassium may reduce growth. | |
| Sulfur | Deficiency | Initial yellowing of young leaves spreading to the whole plant; similar symptoms to nitrogen deficiency but occurs on new growth. |
| Excess | Excess of sulfur may cause premature dropping of leaves. | |
| Iron | Deficiency | Initial distinct yellow or white areas between veins of young leaves lead to spots of dead leaf tissue. |
| Excess | Possible bronzing of leaves with tiny brown spots. | |
| Manganese | Deficiency | Interveinal yellowing or mottling of young leaves. |
| Excess | Older leaves have brown spots surrounded by a chlorotic circle or zone. | |
| Zinc | Deficiency | Interveinal yellowing on young leaves; reduced leaf size. |
| Excess | Excess zinc may cause iron deficiency in some plants. | |
| Boron | Deficiency | Death of growing points arid deformation of leaves with areas of discoloration. |
| Excess | Leaf tips become yellow followed by necrosis. Leaves get a scorched appearance and later fall off. |
Magnesium Deficiency Symptoms
Magnesium ‘is absorbed as divalent Mg2+. In its absence, chlorosis of the older leaves is the first symptom. This chlorosis is usually present between the veins.
The mesophyll cells next to the vascular bundles retain chlorophyll for longer periods than do the parenchyma cells. Magnesium is rarely limiting to plant growth in soils.
Besides its presence in chlorophyll magnesium is essential because it combines with ATP (thereby allowing ATP to function in many reactions) and because it activates many enzymes needed in photosynthesis, respiration, and formation of DNA and RNA.
Calcium Deficiency Symptoms
Calcium is absorbed as a divalent Ca2+. Most soils contain enough Ca2+ for adequate plant growth, but acidic soils where high rainfall occurs are often fertilized with lime (a mixture of CaO and CaCO3) to raise the pH.
In contrast to Mg2+, Ca2+ apparently cannot be loaded into translocating phloem cells. This resulted in a deficiency of calcium.
As a result, deficiency symptoms are always more pronounced in young tissues i.e. (Kirkby and Pilbeam, 1984) meristematic zones of roots, stems, and leaves, where cell divisions occur, are most susceptible because calcium is required to form new middle lamella in the cell plate between daughter cells.
Twisted and deformed tissues result from calcium deficiency, and the meristematic zones die early. In tomatoes, degeneration of young fruits near the blossom “blossom end rot” is caused by calcium deficiency.
Calcium is essential for normal membrane functions in all cells, probably as a binder of phospholipids to each other or membrane proteins. Most calcium in plants is in central vacuoles and bound in cell walls to pectate polysaccharides.
In vacuoles, calcium is frequently precipitated as insoluble crystals of oxalates, and in some species as insoluble carbonate, phosphate, or sulfate. Within the cytosol, much calcium becomes reversibly bound to a small protein called calmodulin.
The structure of calmodulin is altered/changed due to the binding in such a way that it then activates several enzymes. An enzyme-activating role for Ca2+ likely exists mainly when the ion is bound to calmodulin or closely related proteins.
Iron Deficiency Symptoms
Iron-deficient plants are characterized by the development of a pronounced interveinal chlorosis. interveinal chlorosis is due to the nutrient deficiency of magnesium but occurs first on the youngest leaves.
Interveinal chlorosis is sometimes followed by chlorosis of the veins, so the whole leaf then becomes yellow. In severe cases, the young leaves even become white with necrotic lesions.
The reason that iron deficiency results in a rapid inhibition of chlorophyll formation is incompletely known, but two or three enzymes that catalyze certain reactions of chlorophyll synthesis require Fe2+.
The iron that has accumulated in older leaves is relatively immobile in the phloem, as it is in the soil, perhaps because it is internally precipitated in leaf cells as an insoluble oxide or in the form of inorganic or organic ferric-phosphate compounds.
The abundant and stable form of iron in leaves is stored in chloroplasts as in an iron-protein complex called phytoferritin.
Iron deficiencies are often found in particularly sensitive species in the Rose family, including shrubs and fruit trees, and Maize and Sorghum.
The high pH and the presence of bicarbonates contribute to iron deficiency, whereas in acidic soil soluble aluminum is more abundant and restricts iron absorption.
Iron is essential because it forms parts of certain enzymes and numerous proteins that carry electrons during photosynthesis and respiration.
It undergoes alternate oxidation and reduction between the Fe2+ and Fe2+ states as it acts as an electron carrier in proteins.
Chlorine Deficiency Symptoms
Chlorine is absorbed from soil as the chloride ion (Cl–) and most of it remains in this form, although more than 130 organic compounds that contain chlorine have been detected in the plant kingdom in trace amounts.
One of the more interesting is 4-chloroindoleacetic acid, which seems to be a natural auxin hormone. Most species absorb 10 to 100 times as much chloride as they require, so it represents a common example of luxury consumption.
One function of chloride is to stimulate the split (oxidation) of H2O during photosynthesis, but it is also essential for roots, cell division in leaves, and as an important osmotically active solute.
Chloride-deficiency symptoms in leaves consist of reduced growth, wilting, and the development of chlorotic and necrotic spots.
Leaves often eventually attain a bronze color. Roots become stunted in length but thickened, or club-shaped, near the tips.
Chloride is rarely if ever deficient because of its high solubility and availability in soils and because it is also transported in dust or tiny moisture droplets by wind and rain to leaves, where absorption occurs.
Manganese Deficiency Symptoms
Manganese exists in three oxidation states (Mn2+, Mn3+, and Mn4+ as insoluble oxides in soils, and it also exists in chelated form.
It is absorbed largely as the divalent manganous cation- (Mn2+) after either release from chelates or reduction in higher-valence oxides at the root surface.
Deficiencies of manganese are not common, although various disorders such as, “gray speck” of oats, “marsh spot” of peas, and “speckled yellows” of sugar beets result when inadequate amounts are present.
Initial symptoms are often interveinal chlorosis on younger or older leaves, depending on the species, followed by or associated with necrotic lesions.
Electron microscopy of chloroplasts from spinach leaves showed that the absence of manganese causes disorganization of thylakoid membranes but has little effect on the structure of nuclei and mitochondria.
This and much biochemical work indicate that the element plays a structural role in the chloroplast membrane system and that one of its important roles is, like that of chloride, in the photosynthetic split of H2O. The Mn2+ ion also activates numerous enzymes.
Boron Deficiency Symptoms
Boron is almost entirely absorbed from soils as undissociated boric acid (H3BO3, more accurately represented as B(OH)3. It is only slowly translocated out of organs in the phloem of many species once it arrives there in the xylem.
However, in some species, it moves out of the phloem much more effectively. Nutrient deficiency is not common in most areas, yet several disorders are related to the disintegration of internal tissues.
Such as “heart rot” of beets, “stem crack” of celery, “water core” of turnip, and “drought spot” of apples, resulting from an inadequate boron supply.
Plants deficient in boron show a wide variety of symptoms, depending upon the species and plant age. But the earliest symptom is the failure of root tips to elongate normally, accompanied by inhibited synthesis of DNA and RNA.
Cell division in the shoot apex is also inhibited, as is that in young leaves. In the elongation of pollen tubes, the element which plays an undetermined but essential role is boron.
Much evidence indicates that it is required only for two major taxonomic groups, vascular plants, and diatoms. In diatoms, it forms part of the silicon-rich cell wall.
Zinc Deficiency Symptoms
Zinc is absorbed as divalent Zn2+, probably often from zinc chelates. Disorders caused by zinc nutrient deficiency include “little leaf” and “rosette” of apples, peaches, and pecans, resulting from growth reduction of young leaves and stem internodes. Leaf margins are often distorted.
Interveinal Chlorosis
A type of nutrient deficiency in which the leaves from in between the veins become yellowish but the veins themselves remain green.
This is known as interveinal chlorosis and it often occurs in leaves of maize, sorghum, beans, and fruit trees, suggesting that zinc participates in chlorophyll formation or prevents chlorophyll destruction.
The retardation of stem growth in its absence might result partly from its apparent requirement for the production of a growth hormone, indole acetic acid (IAA).
Many enzymes contain tightly bound zinc which is essential for their function. Considering all organisms, more than 80 such enzymes are known.
Copper Deficiency Symptoms
Plants are rarely deficient in copper, partly because they need so little of it. Without copper, young leaves often become dark green and are twisted or otherwise misshapen, often exhibiting necrotic spots.
Citrus orchards are occasionally deficient, in which case the dying young leaves inspired the name “dieback” disease. Copper is absorbed both as the divalent cupric (Cu2+) ion in aerated soils or as the monovalent cuprous ion in wet soil with little oxygen.
Divalent Cu2+ is chelated in various soil compounds, and these likely provide most copper to root surfaces. Partly because such small amounts are needed by plants, copper readily becomes toxic in solution culture unless its amount is carefully controlled.
Copper is present in several enzymes or proteins involved in oxidation and reduction. Two notable examples are cytochrome oxidase, a respiratory enzyme in mitochondria, and plastocyanin, a chloroplast protein.
Molybdenum Deficiency Symptoms
Molybdenum exists to a large extent in soils as molybdate (MoO42-) salts and as MoS2. In the former, molybdenum exists in the redox (valence) state of M06+ but in sulfide salts, it occurs as Mo4+.
Probably because only trace amounts are required by plants, virtually nothing is known about the forms in which it is absorbed and the ways it is changed in plant cells.
Most plants require less molybdenum than any other element, so molybdenum deficiencies are rare. Nevertheless, they are geographically widespread.
Examples of disorders caused by inadequate molybdenum include “whiptail” of cauliflower. Symptoms often consist of interveinal chlorosis occurring first on the older or mid stem leaves, then progressing to the youngest leaves.
Sometimes, as in the “whiptail” disease, plants do not become chlorotic but develop severely twisted young leaves, which eventually die.
In acidic soils; adding lime increases the availability of molybdenum and eliminates or reduces the severity of its deficiency.
The most well-documented function of molybdenum in plants is as part of the enzyme nitrate reductase, which reduces nitrate ions to nitrite ions.
But it may also play a role in the breakdown of purines such as adenine and guanine because of its essentiality as part of the enzyme xanthine dehydrogenase.
A third probable function of molybdenum is to form an essential part of an oxidase that converts abscisic acid aldehyde to the hormone Abscisic acid (ABA).
Nickel in Plants
There is now good evidence that nickel (Ni2+) is an essential element for plants as reported by Dalton et. al., in 1988.
It has been known for several years that nickel is an essential part of an enzyme called urease, which catalyzes hydrolysis (breakdown using H2O) of urea to CO2 and NH4+.
If urease is essential to plants, then nickel would be deemed essential according to the second criterion for essentiality.