Nitrogen Fixation | Mechanism, Types, Nitrogen Cycle 2024
Table of Contents
Nitrogen fixation is the process of reduction of N2 to NH4+ which always appears to be carried on by prokaryotic organisms.
The most abundant supply of nitrogen is found in the earth’s crust, rocks, and sediment (17.5 to 18.4 x 1015 tons); the second-largest reservoir of molecular nitrogen (N2) in the atmosphere (3.5 to 4.0 x 1015 tons).
However, only a relatively few plants are capable of fixing or assimilating, this plentiful supply of nitrogen, and these plants are confined to the lower forms, such as certain groups of bacteria and blue-green algae.
Although higher plants are not able to utilize molecular nitrogen directly, some can utilize it indirectly through the mediation of microorganisms i.e., bacteria in the soil.
As discussed before molecular nitrogen (N2, or atmosphere nitrogen) cannot be used by most plants, it must be converted to nitrate (NO3–), ammonia (NH3), or ammonium (NH4+, the cationic form of NH3).
Asymbiotic Nitrogen Fixation in Plants
The conversion of N2 to NH4+ is accomplished by asymbiotic nitrogen fixation. Molecular nitrogen may also be incorporated into amino acids by symbiotic nitrogen fixation.
Discovery of Nitrogen Fixation Bacteria
Jodin, in 1862, was the first to demonstrate a loss of atmospheric nitrogen and oxygen in a closed system containing a non-sterile solution and a source of carbon.
In 1885 Berthelot demonstrated that the content of fixed nitrogen in non-sterile soil samples increase over some time.
However, credit for actually showing that a living organism is involved in nitrogen fixation goes to Winogradsky, who, in 1894, isolated the anaerobic nitrogen fixation bacteria Clostridium pasorianum.
Two other, even more important, free-living nitrogen-fixing organisms were isolated by Beijerinck in 1909.
In contrast to the anaerobic C. pastorianum, the two nitrogen fixation bacteria isolated by Beijerinck, Azotobacter chroococeum and Azotobacter agile, are aerobic.
Since that time, several nitrogen-fixing species of Azotobacter have been found. Free nitrogen can also be fixed by a large number of blue-green algae.
Symbiotic Nitrogen Fixation in Plants
A relatively large group of plants, the legumes, obtain fixed nitrogen through a symbiotic association with soil bacteria of the genus Rhizobium.
In alder, the symbiotic relationship is with certain species of the genus Actinomyces. In both cases, neither organism alone can fix nitrogen.
The symbiotic relationship between legumes and Rhizobium seems to be species-specific. When certain species of Rhizobium infect legumes, there is no evidence of nitrogen fixation.
Nodulation in Legumes
The actual site of nitrogen fixation is in the nodules formed in the roots of the legume plant as a result of the penetration of rhizobia.
Through Nodulation, the microorganisms provide the host plant with fixed (reduced) nitrogen, and the host plant provides the microorganisms with soluble carbohydrates.
This accumulation probably occurs because plant roots excrete certain growth factors into the soil. Then the bacteria either penetrate the relatively soft root hair tip or invade damaged or broken root hairs and progress in an infection thread through the cortex tissue to the immediate area of the endodermis and pericycle.
Cell divisions commence, in the endodermis and pericycle area, and the nodule grows rapidly and pushes its way to the surface of the root Fig. 11.2.
The factor or factors causing the profuse growth of the cell that form the nodules is at present unknown.
Rhizobia are known to produce the plant hormone indole acetic acid (IAA). However, many other soil microorganisms can produce IAA but are not able to cause nodule formation.
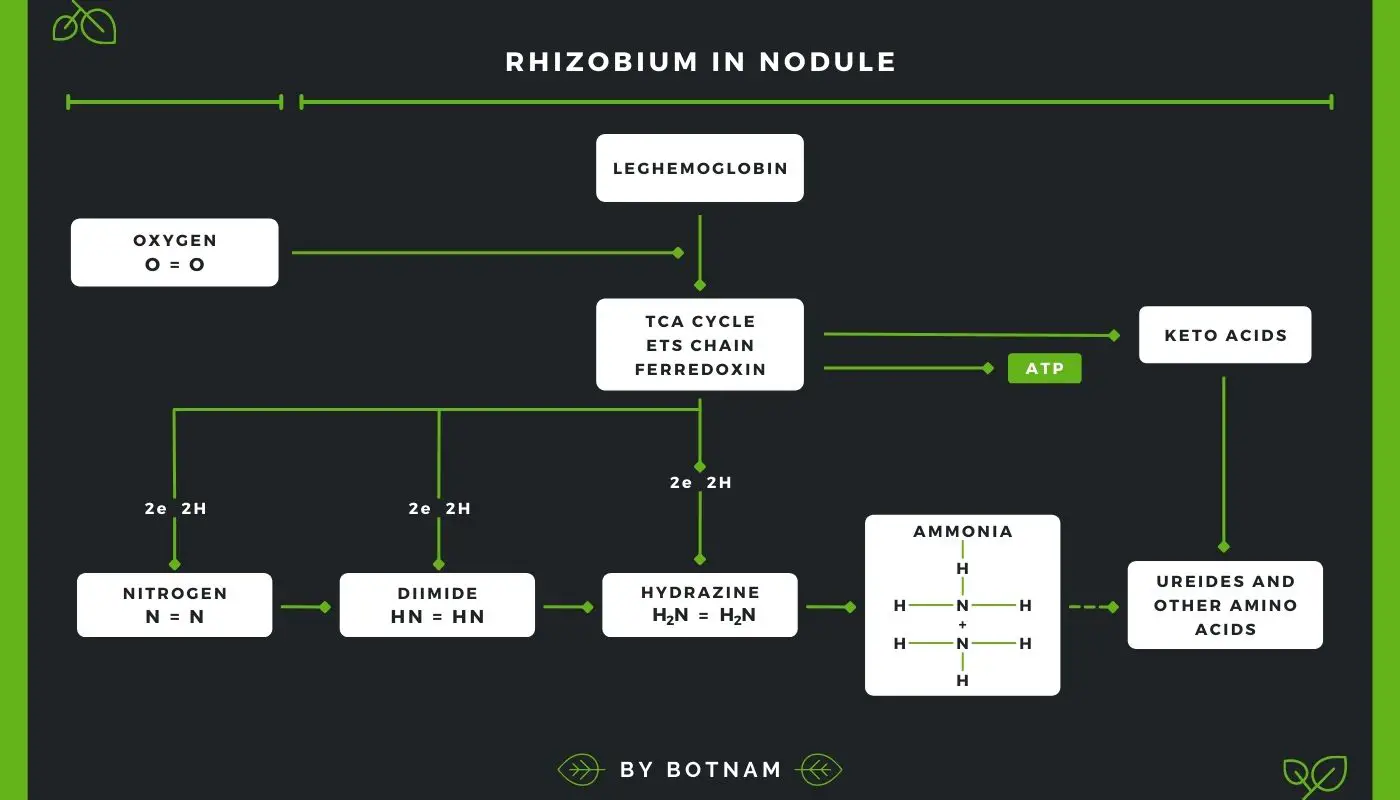
Role of Leghemoglobin
It has been demonstrated that dissection of root nodule reveals the presence of a red pigment which is very similar to the hemoglobin molecule thus it is known as Leghemoglobin as it is a product of Rhizobium legume complex.
It is not found in either (Rhizobium or legume) when growing alone. It has been observed that nodules that lack leghemoglobin cannot fix nitrogen. The concentration of this pigment is directly related to the presence of fixed nitrogen.
Leghemoglobin is an O2 carrier. Oxygen is necessary for the electron transport carrier of the Rhizobium bacteroid. Because of leghemoglobin’s very high affinity for oxygen, it provides oxygen to the root nodule bacteria quickly.
]The keeps the level of molecular oxygen low in bacteroids. This is important because nitrogenase is very sensitive to O2 and cannot function in its presence. Thus, the nodules which lack leghemoglobin cannot fix nitrogen.
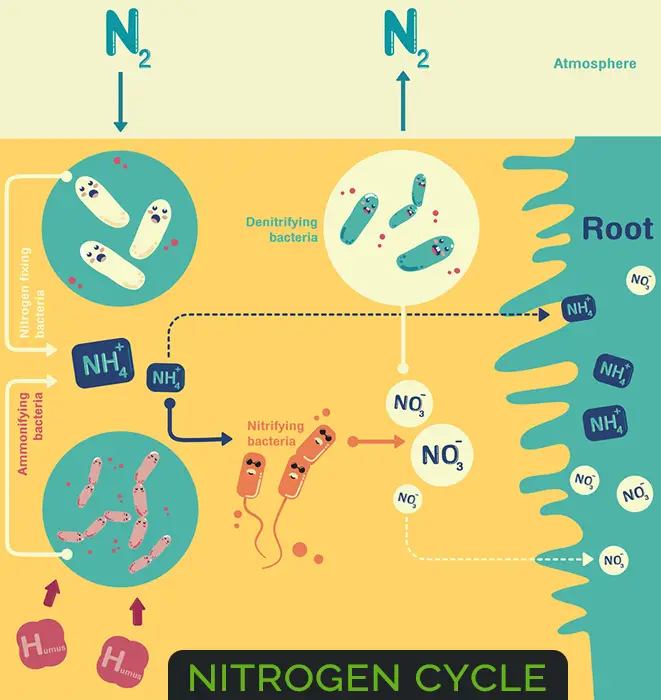
Nitrogen Cycle
Ammonification
The process by which the organic nitrogen is converted into ammonia by bacteria is called ammonification. The reduction of nitrogen to ammonia is catalyzed by a complex of enzymes known as nitrogenase.
Certain micronutrients such as iron, copper, cobalt, and molybdenum appear to be essential. The cobalt requirement has been demonstrated only in the plants capable of fixing molecular nitrogen.
In the case of combined nitrogen cobalt is not required. Molybdenum functions alternately as an electron acceptor and donor in the reduction of nitrogen to ammonia. N2 is reduced to diimide (HN=NH), hydrazine (NH2-NH2), and then ammonia (NH3) (Fig., 1 1.3).
Nitrification (Converters Of Nitrogen In Soil)
Nitrification Definition: The conversion of ammonia to nitrite and then to nitrate is called nitrification. Both Nitrosomonas and Nitrobacter were isolated in 1891 by Sergei Winogradsky.
He demonstrated that Nitrosomonas could convert ammonia only as far as nitrite and that Nitrobacter was needed for the further conversion of nitrite to nitrate.
Process Of Nitrification
Oxidation of ammonia to nitrate in the soil may occur through the mediation of two groups of bacteria: Nitrosomonas and Nitrobacter.
The energy needed for the growth of these organisms is obtained through the oxidation of ammonia or nitrite – in other words, Nitrosomonas and Nitrobacter are autotrophic bacteria and require only inorganic materials for growth, with only one major difference, this type of growth is similar to that found in green plants.
In green plants, light provides the energy for growth, in the bacteria of nitrification, oxidation of ammonia or nitrite provides this energy.
Denitrification
The conversion of nitrate to nitrous oxide (N2O) and nitrogen gas also takes place through the mediation of a variety of soil organisms and is known as denitrification.
The process of denitrification which ends in the release of nitrogen gas into the atmosphere completes nature’s complex nitrogen cycle.
Small amounts of fixed nitrogen are contributed to the soil from electrically produced nitrogen oxides, which are washed down from the atmosphere during rainstorms.
Much greater amounts of fixed nitrogen are contributed by the molecular nitrogen-fixed nitrogen and converted to the many different organic nitrogen compounds of the plant.
This organic nitrogen also contributes to nitrogen development in animals since the animals are unable to convert inorganic nitrogen to organic nitrogen and therefore take it as their diet.